Molar weight
Calculate the weight of 2.00 moles of common salt (NaCl) in grams. M (NaCl) = 58.44 g/mol.
Final Answer:
You need to know the following knowledge to solve this word math problem:
Units of physical quantitiesthemes, topicsGrade of the word problem
Related math problems and questions:
- Euclidean distance
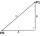 Calculate the Euclidean distance between shops A, B, and C, where: A 45 0.05 B 60 0.05 C 52 0.09 The first figure is the weight in grams of bread, and the second figure is the USD price.
Calculate the Euclidean distance between shops A, B, and C, where: A 45 0.05 B 60 0.05 C 52 0.09 The first figure is the weight in grams of bread, and the second figure is the USD price. - Crystal water
 The chemist wanted to check the water content of the crystallization of chromic potassium alum K2SO4 * Cr2 (SO4) 3 * 24 H2O, which took a long time in the laboratory. From 96.8 g of K2SO
The chemist wanted to check the water content of the crystallization of chromic potassium alum K2SO4 * Cr2 (SO4) 3 * 24 H2O, which took a long time in the laboratory. From 96.8 g of K2SO - Spruce wood
 Calculate the weight of an edge made of spruce wood 6m long when the cross-section of the edge is 146cm square and if the density of the wood is 0.55 grams/cm cubic.
Calculate the weight of an edge made of spruce wood 6m long when the cross-section of the edge is 146cm square and if the density of the wood is 0.55 grams/cm cubic. - Tin body mass
 The tin body transferred 150,000 J of heat, and its temperature dropped by 10°C. Calculate the mass of the tin body. (c of tin is 0.227 kJ/kg. °C). Enter the resulting weight in grams and round it to two decimal places.
The tin body transferred 150,000 J of heat, and its temperature dropped by 10°C. Calculate the mass of the tin body. (c of tin is 0.227 kJ/kg. °C). Enter the resulting weight in grams and round it to two decimal places. - Water material amount
 Calculate the material amount of a 1 kg water sample. M (H 2 O) = 18 g / mol.
Calculate the material amount of a 1 kg water sample. M (H 2 O) = 18 g / mol. - Grams and kilos
 1. Tunji weighs 67 kg 114 g, while Deji weighs 54kg 596g. What is the sum total of their weight? 2. Adamu is 2kg 324g lighter than Muazu; if Muazu weighs 49kg 158g, what is Adamu's weight?
1. Tunji weighs 67 kg 114 g, while Deji weighs 54kg 596g. What is the sum total of their weight? 2. Adamu is 2kg 324g lighter than Muazu; if Muazu weighs 49kg 158g, what is Adamu's weight? - Weight fraction
 To prepare the brine, we need 1500 g of an aqueous salt solution with a weight fraction of 0.08. What salt weight and water volume do we need to prepare it?
To prepare the brine, we need 1500 g of an aqueous salt solution with a weight fraction of 0.08. What salt weight and water volume do we need to prepare it?
