Themes, topics - math word problems - page 15 of 168
Number of problems found: 3360
- Missile 3
 The missile penetrated the embankment to a depth of 1.2 m. What was its speed when it hit the surface of the embankment if the movement of the missile in the ground lasted 0.021 seconds and if the movement was uniformly slowed down?
The missile penetrated the embankment to a depth of 1.2 m. What was its speed when it hit the surface of the embankment if the movement of the missile in the ground lasted 0.021 seconds and if the movement was uniformly slowed down? - Divisible PIN
 Marek's mother helped him with his shopping. She wanted to pay with a card when collecting the goods in person, but she wasn't sure what her PIN was. Could it have been if her PIN code was a number divisible by six?
Marek's mother helped him with his shopping. She wanted to pay with a card when collecting the goods in person, but she wasn't sure what her PIN was. Could it have been if her PIN code was a number divisible by six? - Digit sum MO Z9-I-4
 Determine whether it is possible to add a single-digit number to the number with the digit sum of 2024 so that the resulting number has the digit sum of 74.
Determine whether it is possible to add a single-digit number to the number with the digit sum of 2024 so that the resulting number has the digit sum of 74. - Semicircles
 In a rectangle with sides of 4cm and 8cm, there are two different semicircles, each of which has its endpoints at its adjacent vertices and touches the opposite side. Construct a square such that its two vertices lie on one semicircle, the remaining two o
In a rectangle with sides of 4cm and 8cm, there are two different semicircles, each of which has its endpoints at its adjacent vertices and touches the opposite side. Construct a square such that its two vertices lie on one semicircle, the remaining two o - Four-digit numbers
 Find all four-digit numbers whose decimal notation begins with a six and ends with a two and is divisible by 24.
Find all four-digit numbers whose decimal notation begins with a six and ends with a two and is divisible by 24. - Acceleration of a car
 What was the initial speed of the car when, after accelerating 2 m/s², it gained a speed of 30m/s in 10 seconds?
What was the initial speed of the car when, after accelerating 2 m/s², it gained a speed of 30m/s in 10 seconds? - Acceleration - free fall
 How long will it take for a car to reach speed of 180 km/h with an acceleration of 10 m/s²?
How long will it take for a car to reach speed of 180 km/h with an acceleration of 10 m/s²? - Distance Under Acceleration
 What distance will a car travel with an acceleration of 10m/s² in 5 seconds?
What distance will a car travel with an acceleration of 10m/s² in 5 seconds? - Two cubes 3
 Two cubes modeled from plasticine have single-digit integer edge lengths with a difference of 1 cm. Can one large cube with an integer edge length be modeled from the same amount of plasticine?
Two cubes modeled from plasticine have single-digit integer edge lengths with a difference of 1 cm. Can one large cube with an integer edge length be modeled from the same amount of plasticine? - Four-digit - sum
 A four-digit number has a digit sum of 20. The sum of its last two digits is equal to the second digit increased by 5. The sum of the outer digits is equal to the second digit decreased by 3. If we write the digits of this number in reverse order, the num
A four-digit number has a digit sum of 20. The sum of its last two digits is equal to the second digit increased by 5. The sum of the outer digits is equal to the second digit decreased by 3. If we write the digits of this number in reverse order, the num - Two resistors 5
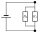 Two resistors are connected in parallel. The first resistor has a resistance of 2000 ohms and a current of 4 mA flows through it. The second resistor has a resistance of 1600 ohms. 1/Draw a circuit diagram 2/Determine the voltage of the power source. 3/Wh
Two resistors are connected in parallel. The first resistor has a resistance of 2000 ohms and a current of 4 mA flows through it. The second resistor has a resistance of 1600 ohms. 1/Draw a circuit diagram 2/Determine the voltage of the power source. 3/Wh - To the city
 A bus leaves for a city 54 km away at an average speed of 15 m/s. 12 minutes after the bus leaves, a motorcycle leaves from the same place to follow it. Calculate the average speed of the motorcycle when it arrives at the destination at the same time as t
A bus leaves for a city 54 km away at an average speed of 15 m/s. 12 minutes after the bus leaves, a motorcycle leaves from the same place to follow it. Calculate the average speed of the motorcycle when it arrives at the destination at the same time as t - Tractor 17
 The tractor has an average speed of 2.4 km/h in the first minute of starting, 3.6 km/h in the second and third minutes, and 5.0 km/h in the fourth to sixth minutes. What movement did it make during the first six minutes of driving? Determine the average s
The tractor has an average speed of 2.4 km/h in the first minute of starting, 3.6 km/h in the second and third minutes, and 5.0 km/h in the fourth to sixth minutes. What movement did it make during the first six minutes of driving? Determine the average s - Laboratory
 In the laboratory, twelve liters of 80% prepared 40% alcohol were diluted to a final 70% alcohol. A five-liter barrel of 40% alcohol was available. How much will remain in the barrel of 40% alcohol?
In the laboratory, twelve liters of 80% prepared 40% alcohol were diluted to a final 70% alcohol. A five-liter barrel of 40% alcohol was available. How much will remain in the barrel of 40% alcohol? - Future Value with Interest
 The deposit is €1000. The interest is 7.36% per year. What is the amount after 3 years and 9 months?
The deposit is €1000. The interest is 7.36% per year. What is the amount after 3 years and 9 months? - Distance - cities
 Determine the actual distance between two cities if their distance on the map is 7.5 cm and the map scale is 1:60,000.
Determine the actual distance between two cities if their distance on the map is 7.5 cm and the map scale is 1:60,000. - Timetable
 If a train traveling between two cities increases the speed specified in the timetable by 5 km/h, it will arrive 20 minutes earlier. If it decreases it by 5 km/h, it will arrive 25 minutes later. How long is the route between the cities?
If a train traveling between two cities increases the speed specified in the timetable by 5 km/h, it will arrive 20 minutes earlier. If it decreases it by 5 km/h, it will arrive 25 minutes later. How long is the route between the cities? - Vertical movement
 Find the length of the vertical path you must travel to lift a weight with a force of 0.01 N to do 1 J of work.
Find the length of the vertical path you must travel to lift a weight with a force of 0.01 N to do 1 J of work. - Aluminum - density
 The density of aluminum is 2,700 kg/m³. Calculate the mass of an aluminum cube with a volume of 80 cm3!
The density of aluminum is 2,700 kg/m³. Calculate the mass of an aluminum cube with a volume of 80 cm3! - Aluminum ignote
 At what temperature did the temperature stabilize if an aluminum ignote weighing 45 kg and at a temperature of 15°C received 4.8 MJ of heat? The specific heat capacity of aluminum is 896 J/kg°C.
At what temperature did the temperature stabilize if an aluminum ignote weighing 45 kg and at a temperature of 15°C received 4.8 MJ of heat? The specific heat capacity of aluminum is 896 J/kg°C.
Do you have unsolved math question and you need help? Ask a question, and we will try to solve it. We solve math question.
