Atmospheric pressure
The measured value of atmospheric pressure is 96,000 Pa. We want to verify this value with a closed tube at one end. Before measuring, fill the tube with glycerol (density of glycerol ρ = 1200 kg/m3). How long does the tube have to be?
Final Answer:
Tips for related online calculators
Tip: Our volume units converter will help you convert volume units.
Tip: Our Density units converter will help you convert density units.
Do you want to convert velocity (speed) units?
Tip: Our Density units converter will help you convert density units.
Do you want to convert velocity (speed) units?
You need to know the following knowledge to solve this word math problem:
solid geometryUnits of physical quantitiesthemes, topicsGrade of the word problem
Related math problems and questions:
- Gas pressure
 The mean square velocity of the gas molecules is 1200 m/s. What pressure does this gas exert on the vessel wall when its density is 0.03 kg/m³?
The mean square velocity of the gas molecules is 1200 m/s. What pressure does this gas exert on the vessel wall when its density is 0.03 kg/m³? - Air bubble
 The air bubble at the bottom of the lake at a depth of h = 21 m has a radius of r1 = 1 cm at a temperature of t1 = 4°C. The bubble rises slowly to the surface, and its volume increases. Calculate its radius when it reaches the lake's surface, with a tempe
The air bubble at the bottom of the lake at a depth of h = 21 m has a radius of r1 = 1 cm at a temperature of t1 = 4°C. The bubble rises slowly to the surface, and its volume increases. Calculate its radius when it reaches the lake's surface, with a tempe - Submerged cork body
 The cork body floats first in water and then in glycerol. The submerged volume of the cork body in water is 0.0006 m3, and in glycerol, it is 0.0005 m³. The density of water is 1000 kg/m3, and the density of glycerol is 1200 kg/m³. C
The cork body floats first in water and then in glycerol. The submerged volume of the cork body in water is 0.0006 m3, and in glycerol, it is 0.0005 m³. The density of water is 1000 kg/m3, and the density of glycerol is 1200 kg/m³. C - Manometer gas pressure
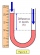 An open manometer is connected to the gas tank. The difference in water levels in both arms is 54.5 cm. The atmospheric pressure is 10 5 Pa. Calculate the gas pressure.
An open manometer is connected to the gas tank. The difference in water levels in both arms is 54.5 cm. The atmospheric pressure is 10 5 Pa. Calculate the gas pressure. - Water tube pressure
 Water flows in a horizontal tube at a speed of 2.24 m/s and has a pressure of 0.1 MPa. How fast does the water flow in the narrowed part of the tube if its pressure is 0.09 MPa?
Water flows in a horizontal tube at a speed of 2.24 m/s and has a pressure of 0.1 MPa. How fast does the water flow in the narrowed part of the tube if its pressure is 0.09 MPa? - Railway wagon
 The railway wagon holds a 75 m³ load. The wagon can carry a maximum weight of 30 tonnes. What is the maximum density that may have material with which we could fill this whole wagon? b) How much peat (density 350 kg/m3) can carry 15 wagons?
The railway wagon holds a 75 m³ load. The wagon can carry a maximum weight of 30 tonnes. What is the maximum density that may have material with which we could fill this whole wagon? b) How much peat (density 350 kg/m3) can carry 15 wagons? - Pool filling time
 How long does it take to fill a pool measuring 6x4x1m at a flow rate of Q = 0.2 dm³ / s?
How long does it take to fill a pool measuring 6x4x1m at a flow rate of Q = 0.2 dm³ / s?
