Fuel efficiency
The gravimetric analysis of an anthracite fuel is 90% carbon, 3% hydrogen, 2% oxygen, and 1% nitrogen. Given that analysis of the dry combustion products by volume is 16.2% CO2, 3.5% O2, and 80.3% N2, during the combustion process of anthracite, which considers a solid fuel, some combustibles exit in fly ash with a weight fraction equal to 19% and temperature 270°C, The fly ash is 15 % of the total ash, and there are losses due to combustibles in slag ash where the combustibles with weight fraction equal 5% and temperature 4500°C. Ignoring the weight fraction of the water in the fuel, take the ambient condition 298K, 0.1MPa. Calculate the combustion efficiency if the weight fraction of ash in fuel is 30%. For anthracite fuel, take the value of the constant K in dry flue gas losses by 0.67 on the gross C.V basis and by 0.68 on the net C.V basis. The heat reaction of CO to that of CO2 equals 65.
Final Answer:

You need to know the following knowledge to solve this word math problem:
Units of physical quantitiesthemes, topicsGrade of the word problem
Related math problems and questions:
- Salami
 We have six kinds of salami, six of which have ten pieces, and one of which has four pieces. How many ways can we distinctly choose five pieces of salami?
We have six kinds of salami, six of which have ten pieces, and one of which has four pieces. How many ways can we distinctly choose five pieces of salami? - Std-deviation
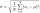 Calculate the standard deviation for file: 63,65,68,69,69,72,75,76,77,79,79,80,82,83,84,88,90
Calculate the standard deviation for file: 63,65,68,69,69,72,75,76,77,79,79,80,82,83,84,88,90 - Electric cooker
 During which time does an electric cooker with power input P = 500 W and efficiency n = 75% heat water with mass m = 2 kg and temperature t1 = 10°C to the boiling point (t2 = 100°C)? The specific heat capacity of water is c = 4 180 J/kg/K.
During which time does an electric cooker with power input P = 500 W and efficiency n = 75% heat water with mass m = 2 kg and temperature t1 = 10°C to the boiling point (t2 = 100°C)? The specific heat capacity of water is c = 4 180 J/kg/K. - Test scores
 Below is a collection of test scores from a class of 20 students. Make 2 histograms of the data. Choose your own horizontal scales as long as you have more than 4 cells in each histogram. 65 70 68 87 98 91 77 85 70 72 86 86 94 95 67 88 77 99 74 71
Below is a collection of test scores from a class of 20 students. Make 2 histograms of the data. Choose your own horizontal scales as long as you have more than 4 cells in each histogram. 65 70 68 87 98 91 77 85 70 72 86 86 94 95 67 88 77 99 74 71 - Petra
 Petra likes to bathe in a bathtub with a water temperature of 38 degrees. She turned on the hot water set at 70 degrees and left for a moment. Before she returned, 40 liters of water flowed into the tub. How much water with a temperature of 22 degrees mus
Petra likes to bathe in a bathtub with a water temperature of 38 degrees. She turned on the hot water set at 70 degrees and left for a moment. Before she returned, 40 liters of water flowed into the tub. How much water with a temperature of 22 degrees mus - Speed of Slovakian trains
 Rudolf took the train from the station 'Nitra' to 'Nové Zámky'. In the train timetables found train Os 5004 : km 0 Prievidza 14:25 4 Koš 14:30 14:31 9 Nováky 14:36 14:37 13 Zemianske Kostoľany 14:42 14:43 16 Bystričany 14:47 14:48 19 Oslany 14:51 14:51 23
Rudolf took the train from the station 'Nitra' to 'Nové Zámky'. In the train timetables found train Os 5004 : km 0 Prievidza 14:25 4 Koš 14:30 14:31 9 Nováky 14:36 14:37 13 Zemianske Kostoľany 14:42 14:43 16 Bystričany 14:47 14:48 19 Oslany 14:51 14:51 23 - By mass
 How much heat must we supply to completely convert potassium weighing 45 kg at a temperature of 22.4 °C into potassium at a temperature of 98 °C? The specific heat of fusion is 25.5 kJ/kg, the melting point is 63.32 °C. The specific heat capacity of potas
How much heat must we supply to completely convert potassium weighing 45 kg at a temperature of 22.4 °C into potassium at a temperature of 98 °C? The specific heat of fusion is 25.5 kJ/kg, the melting point is 63.32 °C. The specific heat capacity of potas
